Carbohydrates are the most abundant biomolecules on Earth, come in a variety of flavours and play a multitude of roles:
- sugar and starch are dietary staples in most parts of the world
- insoluble carbohydrates serve as structural and protective elements in the cell walls of bacteria and plants and in the connective tissues of animals
- other carbohydratess lubricate skeletal joints and participate in recognition of and adhesion between cells
- glycoconjugates are complex carbohydrate polymers covalently attached to proteins or lipids and act as signals that determine the intracellular location or metabolic fate of these hybrid molecules
Did you know that the oxidation of carbohydrates is the central energy-yielding pathway in most non-photosynthetic cells?
There are three major size classes of carbohydrates: monosaccharides, oligosaccharides, and polysaccharides, depending on the number of aldehyde or ketone units present in the molecule. Most carbohydrates have the chemical formula (CH2O)n, which means they have a ratio of 1:2:1 of molecules of carbon, hydrogen and oxygen; some also contain nitrogen, phosphorus, or sulfur.
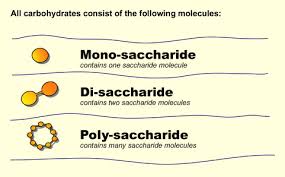
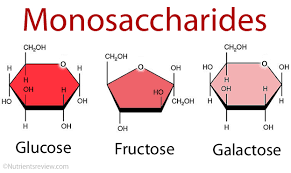
The most abundant monosaccharide in nature is the six-carbon sugar D-glucose, sometimes referred to as dextrose. Other prevalent monosaccharides are:
- glyceraldehyde, a three-carbon monosaccharide and one of the simplest
- erythrose, a four-carbon monosaccharide
- ribose, a five-carbon monosaccharide
- fructose and galactose, which are six-carbon monosaccharides
Oligosaccharides consist of short chains of monosaccharides, the most abundant of which are the disaccharides, with two such monosaccharide units. Typical is sucrose or table sugar, which consists of a molecule of glucose linked to a molecule of fructose. Other prevalent disaccharides are maltose (2 linked molecules of glucose), or lactose (a molecule of glucose, linked to a molecule of galactose).

Polysaccharides are polymers containing more than 20 monosaccharide units, and some have hundreds or even thousands of units. Homopolysaccharides contain only a single type of monomer, whereas heteropolysaccharides contain two or more different kinds.
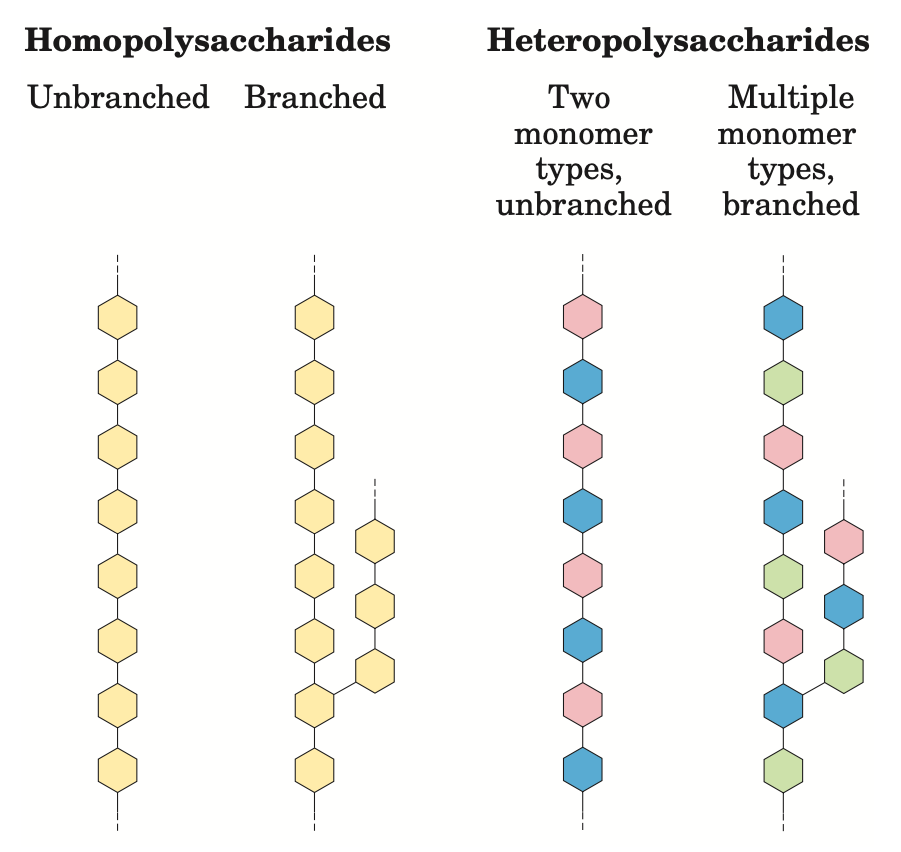
Some polysaccharides, such as cellulose, are linear chains, whereas others, such as glycogen, are branched. Both glycogen and cellulose consist of recurring units of D-glucose, but they differ in the type of glycosidic linkage and consequently have strikingly different properties and biological role.
Did you know that about 100 billion metric tons of carbon dioxide and water are converted yearly through photosynthesis into cellulose and other plant products.
Starch contains two types of glucose polymer, amylose and amylopectin.
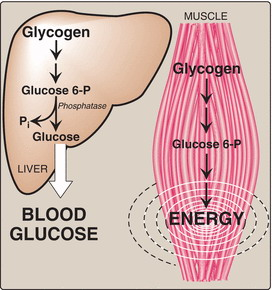
Glycogen is the main storage polysaccharide of animal cells. It is more extensively branched and more compact than starch. Glycogen is especially abundant in the liver, where it may constitute as much as 7% of the wet weight; it is also present in skeletal muscle.
Dextrans are bacterial and yeast polysaccharides.
Cellulose and chitin serve structural roles. Cellulose, a fibrous, tough, water-insoluble substance, is found in the cell walls of plants, particularly in stalks, stems, trunks, and all the woody portions of the plant body. Cellulose constitutes much of the mass of wood, and cotton is almost pure cellulose.

Chitin on the other hand forms extended fibers similar to those of cellulose, and like cellulose cannot be digested by vertebrates. Chitin is the principal component of the hard exoskeletons of arthropods (think insects, lobsters, and crabs) and, is probably the second most abundant polysaccharide in nature, after cellulose.

Examples of heteropolysaccharides are peptidoglycan found in bacterial cell walls, agarose found in algae cell walls and hyaluronic acid, a glycosaminoglycans found in the extracellular matrix of cartilage and tendons, to which it contributes tensile strength and elasticity. Hyaluronates form clear, highly viscous solutions that serve as lubricants in the synovial fluid of joints and give the vitreous humor of the vertebrate eye its jelly- like consistency.
#Carbs #Glucose #Saccharides #Sucrose #Lactose #Glycogen #Starch #NutriFix #FixingNutrition #HealthAndLongevity #SmartEating #HealthyFood #OptimizeYourHealth #NutritionKnowledge #NutritionKnowHow #YouAreWhatYouEat
~Mihai