Proteins are the most abundant biological macromolecules, occurring in a great variety across all types of cells; they range in size from relatively small peptides to huge polymers. Proteins are also the molecules through which genetic information is expressed. They fulfil a diversity of biological functions and are the most important final products of the information pathways.
All proteins, ranging from the most ancient lines of bacteria to the most complex forms of life, are constructed from the same 20 amino acids. These can be joined in many different combinations and sequences to produce proteins with strikingly different properties and activities, such as enzymes, hormones, antibodies, transporters, muscle fibers, the lens protein of the eye, feathers, spider webs, rhinoceros horn, milk proteins, antibiotics, mushroom poisons, etc. Among these protein products, enzymes are the most varied and specialized, as all cellular reactions are catalyzed by them.
The first amino acid to be discovered was asparagine, in 1806 and the last of the 20 to be found, threonine, was not identified until 1938. Names of amino acids have are in some cases derived from the source from which they were first isolated. For example:
- asparagine was first found in asparagus
- glutamate in wheat gluten
- tyrosine was first isolated from cheese (tyros in Greek means “cheese”)
- glycine was so named because of its sweet taste (glykos, means “sweet” in Greek)

All 20 of the common amino acids are alpha-amino acids, meaning that the carboxyl and amino group are bonded to the same carbon atom (the alpha carbon). They differ from each other in their side chains, or R groups, which vary in structure, size, and electric charge, and which influence the solubility of the amino acids in water.
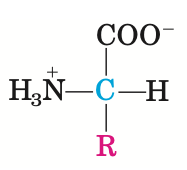
The 9 amino acids that cannot be synthesized by the human body and need to be obtained through nutrition are called essential amino acids or EAAs. These are: isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valin and histidine. Our Oucescu smoothie is chockfull of these..
Amino acids can also be classified depending on their R group into:
- non-polar aliphatic: glycine, alanine, proline, valine, leucine, isoleucine, methionine
- aromatic: phenylalanine, tyrosine, tryptophan
- polar, uncharged: serine, threonine, cysteine, asparagine, glutamine
- polar, positively charged: lysine, histidine, arginine
- polar, negatively charged: aspartate, glutamate
There is also a myriad of less common amino acids, which are modified variants of the 20 common amino acids:
- 4-hydroxyproline and 5-hydroxylysine, derived from proline and lysine and found in plant cell wall proteins and collagen respectively
- 6-N-Methyllysine, a constituent of myosin, one of the contractile proteins of muscle
- alpha-carboxyglutamate, found in the blood-clotting protein prothrombin
- desmosine, a derivative of four lysine residues, found in the fibrous protein elastin
- Ornithine and citrulline, key intermediates in the biosynthesis of arginine and in the urea cycle
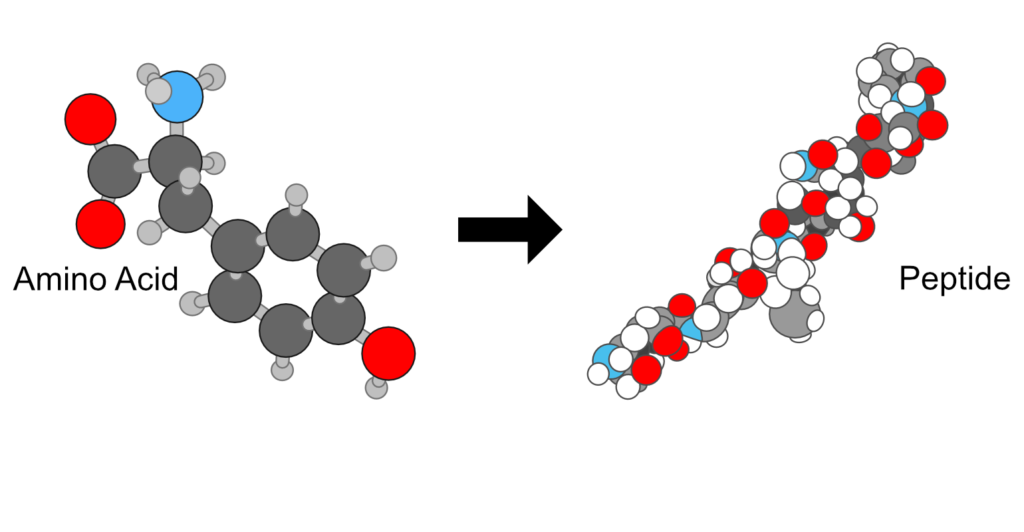
Amino acid molecules can be covalently joined through peptide bonds to yield dipeptides, tripeptides, tetrapeptides, etc, oligopeptides or polypeptides. Proteins are polypeptides with molecular weights higher than 10,000.
Some proteins consist of a single polypeptide chain, but others, called multisubunit proteins, have two or more polypeptides associated noncovalently. Hemoglobin, for example, has four polypeptide subunits: two identical alpha chains and two identical beta chains.
Some proteins, called conjugated proteins, contain in addition to amino acids, permanently associated chemical components, called prosthetic groups, which play important roles in the protein’s biological function. Conjugated proteins are of several types:
- lipoproteins – contain lipids
- glycoproteins – contain sugar groups
- metalloproteins – contain specific metals
Each type of protein has a unique amino-acid sequence and three-dimensional structure; these confer the protein’s unique function.
So get to know the proteins in your body by first adjusting your dietary protein intake. Try out a NutriFix smoothie today!
~Mihai







Pingback: Big fish vs. Small fish – NutriFix
Pingback: Neurotransmitter metabolism – Nutrifix